China Moisturizing Suppliers
Here you can find the related products in Moisturizing, we are professional manufacturer of Moisturizing. We focused on international export product development, production and sales. We have improved quality control processes of Moisturizing to ensure each export qualified product.
If you want to know more about the products in Moisturizing, please click the Product details to view parameters, models, pictures, prices and Other information about Moisturizing.
Whatever you are a group or individual, we will do our best to provide you with accurate and comprehensive message about Moisturizing!
Hyaluronic Acid, Sericin Powder, Polyglutamic Acid, squalane,Vitamin B5,Panthenol Xi'an Gawen Biotechnology Co., Ltd , https://www.ahualyn-bio.com
2017 single anti-drug harvest! Approved new drug highlights, local business success, latest research and development trends... panoramic interpretation
After more than 30 years of accumulation, the pharmaceutical network from monoclonal antibody development, from clinical research to commercialization strategies, has matured in various aspects and has become a well-deserved mainstream branch of the pharmaceutical industry.
Compared with the past few years, the global monoclonal drug field in 2017 is still hot: as of December 31, 2017, the FDA and EMA have approved 10 monoclonal antibody drugs, and the global mainstream market approved monoclonal antibodies. The drug (including drugs that have been withdrawn for various reasons after approval, excluding Fc fusion protein) has reached 73 cumulatively; clinical stages, new projects continue to advance, new targets and new technologies are under concept Verification, step-by-step verification of these concepts will give monoclonal antibody drugs greater potential for application.
It can be said that we are experiencing the golden age of the development of monoclonal antibody drugs. On the occasion of this new and old year, the author tried to sort out the market conditions and research and development of monoclonal antibody drugs in 2017, and made a prospect for the new year that has already begun.
Twenty years of big data: the number of the world's total of 73 approved
The authors summarized the approval of new monoclonal antibody drugs in the global mainstream market (see Figure 1). It can be seen that the total number of approved monoclonal antibody drugs has reached 73. The number of approved antibodies in one year has increased significantly since 2014, and has risen steadily over the next three years. The development of monoclonal antibody drugs has entered a large-scale development period from the early piecemeal stage. In 2017, the global mainstream market was approved for 10 new monoclonal antibody drugs, the highest in history.
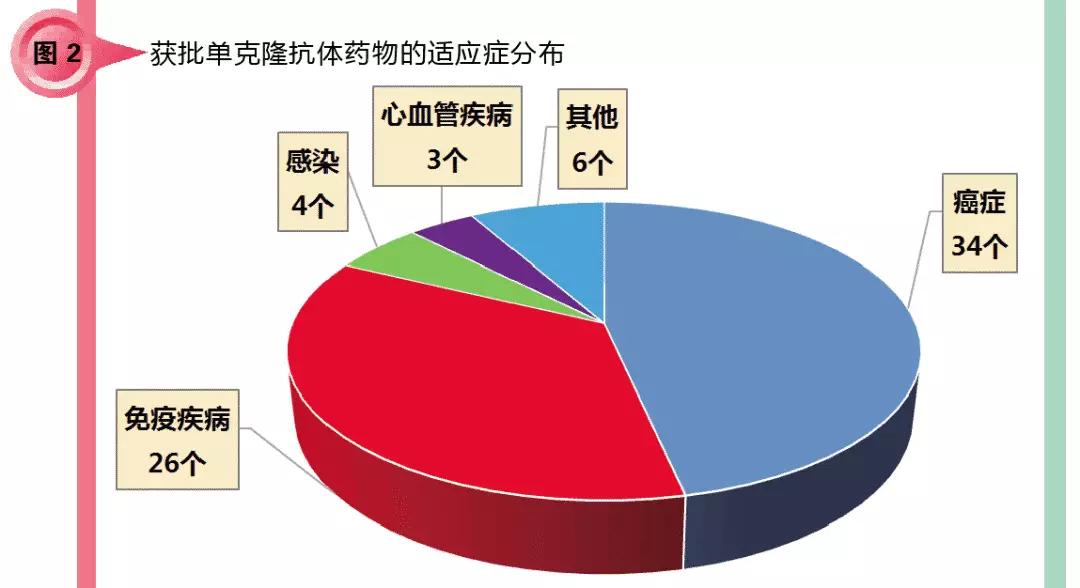
Classification of 73 monoclonal antibody drugs, from the distribution of indications, mainly for cancer (including hematological cancer and non-hematological cancer) and immune diseases (including autoimmune diseases and inflammation caused by external factors) There are 60 antibodies in the disease field, accounting for 82.2% of the total; infection and cardiovascular diseases are also involved, a total of 7 antibodies; another 6 antibodies for the treatment of orthopedic diseases, ophthalmic diseases, rare diseases and so on.
The main reasons for the indications of cancer and immune diseases are because of the large number of patients and the large number of market segments. Secondly, the research on the related mechanisms of these two diseases is relatively mature, and the drug targets are suitable for the development of monoclonal antibody drugs. Other areas account for a relatively small proportion. However, the author believes that with the deepening of basic research and breakthroughs in clinical trials, monoclonal antibody drugs will penetrate more and other fields other than cancer and immune diseases.
Drugs approved in 2017: 10 new highs in the calendar year, and there are many highlights
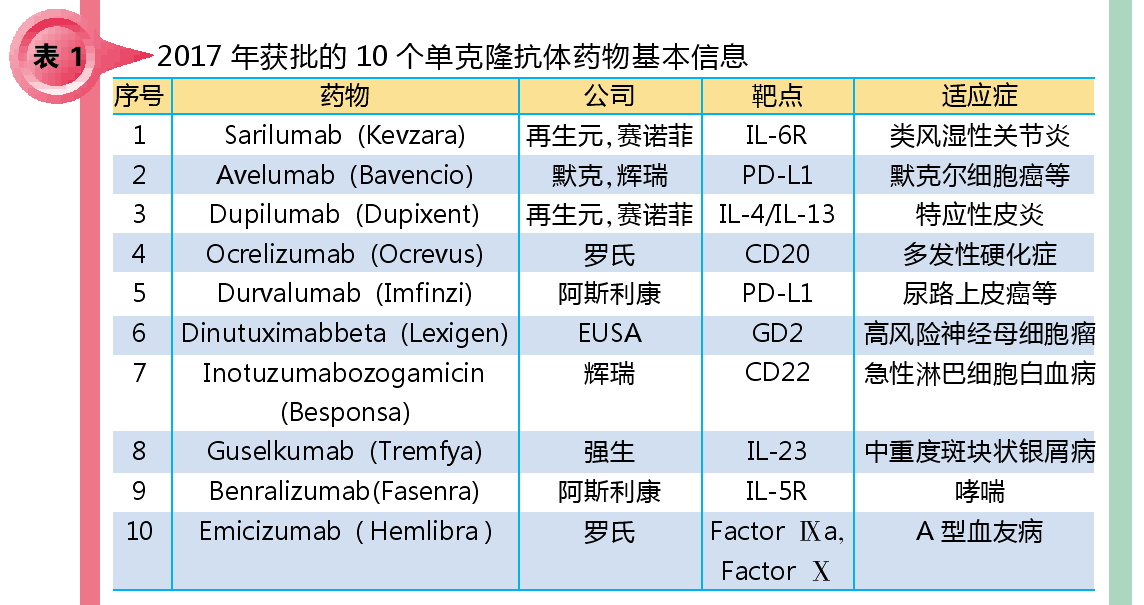
The basic information of the 10 monoclonal antibody drugs approved in 2017 is shown in Table 1.
Sarilumab (Kevzara), developed in collaboration with Sanofi and Regeneration, is a monoclonal antibody targeting the interleukin-6 receptor. It was approved by the FDA in May 2017 and approved by the EMA in June for moderate to severe rheumatoid arthritis. Second-line treatment. The results of the SARIL-RA-MONARCH, a head-to-head phase III clinical study with adalimumab in 2016, showed that Sarilumab was superior to adalimumab, suggesting that adalimumab may experience strong competition.
Both Avelumab (Bavencio) and AstraZeneca's Durvalumab (Imfinzi), developed by Merck and Pfizer, are monoclonal antibodies targeting PD-L1, and their approval and marketing have led to an increase in marketed PD-1/PD-L1 antibodies. To five, the competition in the tumor immune market is further fierce.
Pfizer's Inotuzumabozogamicin (Besponsa) is an antibody drug conjugate (ADC) that allows small molecule toxicants to precisely kill cancer cells that express CD22 with high molecular weight. The drug was approved by the European Union in June 2017 and is the fourth approved ADC. Its approval has increased confidence in the development of this class of drugs.
Roche's Emicizumab (Hemlibra) is the last approved monoclonal antibody drug in 2017. It targets the clotting factors IXa and X, promoting the binding of IXa and X, thereby promoting the blood coagulation process. The launch of Emicizumab can help patients with hemophilia A reduce the frequency of medication and improve their quality of life. At the same time, the drug is also the first approval of bispecific antibodies in the non-cancer field.
It can be seen that the number of approved monoclonal antibody drugs in this year has not only increased, but also has a bright spot.
Late clinical projects: the pattern of "two major categories" of indications has changed
According to the information retrieved by the author, as of December 31, 2017, nearly 60 new drug drugs were in the clinical phase III research phase. From the distribution of indications, although cancer and immune diseases still occupy the majority, the proportion of R&D projects in other disease areas has increased, the most obvious of which is neurological diseases, mainly Alzheimer's disease and partiality. headache.
Alzheimer's disease has not been listed for many years, and the market space is very large. If monoclonal antibody drugs are approved, relevant companies will gain huge profits by filling the market vacancies. However, from the current clinical trial results, it seems that only Biogen's Aducanumab has approved hope.
Migraine is also a long-term unsuitable therapeutic drug, and the market is very large. But unlike Alzheimer's, migraine-related research and development pipelines are progressing well: Amgen's Erenumab (targeting CGRPR), Lilly's Galcanezumab (targeting CGRP), and Teva's Fremanezumab (targeting CGRP) have been submitted The third-phase clinical data of Alder's Eptinezumab (targeted CGRP) is also very active, and the company is also preparing to submit new drug applications in the second half of 2018.
Pharmaceutical companies have also had more layouts in other disease areas such as eye diseases and blood diseases. The penetration of monoclonal antibody drug development into these areas is due to the deepening of basic research and target validation, as well as to the increasingly complex commercial competition.
Technology development and application: structural transformation, screening methods continue to break through
The development and application of the technical aspects of monoclonal antibody drug development are mainly reflected in two aspects: one is the modification of antibody structure, and the other is a new antibody screening method.
In terms of structural modification, the author concludes that there are three categories: bispecific antibodies, antibody drug conjugates (ADC), and miniaturized antibodies.
Bispecific antibodies are no stranger to the world. Many companies and laboratories around the world have different bispecific antibody platforms. There are already three approved bispecific antibody drugs: Catumomamap, Blinatumomab and Emicizumab. The concept of early bispecific antibodies is mainly for cancer treatment. It is hoped that the effect of drugs to kill cancer cells can be enhanced by multi-target and multi-channel inhibition. Later, this concept was gradually extended to other diseases such as immune diseases and anti-infection. . However, although the design can be imagined, the actual clinical effect of the bispecific antibody needs to be more verified, and there are great challenges in production, which requires long-term exploration and optimization.
The core technology of ADC is mainly the design of the linker between the antibody and the small molecule drug and the selection of the attachment site on the antibody. After long-term development, the basic problems have been solved, and ADC technology has gradually entered the stage of large-scale application. At present, there are about 9 ADC drugs under clinical phase III.
Miniaturized antibodies include Nanobodies, small antibody-like scaffold proteins, and the like. The development of this class of drugs is mainly to improve some of the inherent defects of the standard structure of antibodies, including complex screening processes, poor tissue permeability, and high production costs. At present, Ablynx of Belgium is in an absolute leading position in the research and development of nano-antibodies. Through independent research and development and cooperative development, it has already entered several clinical stages; other antibody-like scaffold proteins, such as Affibody and DARPins, are also in concept. The stage of verification.
In terms of screening method development, it is mainly the gradual popularization of humanized mice and the gradual maturity of antibody screening methods based on single B cells. Both of these technologies have undergone a long period of development. Currently, monoclonal antibody drugs obtained by humanized mouse technology have been marketed, but the single B cell antibody screening method has not yet been produced, but it is an obvious Direction of development.
Popular research and development targets: Tumor immune checkpoint inhibitors stand out
Tumor immunotherapy based on immunological checkpoint inhibitors no longer only stays at the level of single-agent targeted therapy, but has become a therapeutic idea to restore or enhance the vitality of the patient's own immune system, using the immune system to kill cancer. cell.
The early development was mainly for the monotherapy of CTLA-4, PD-1, PD-L1 and other immune checkpoints. By preventing the cancer cells from inhibiting the body's own T cells and restoring the tumor killing effect of T cells, a good result was obtained. effect. Now, the mainstream R&D thinking is centered on these immune checkpoint inhibitors to explore a combination of treatments with other drugs.
Several companies are exploring the possibility of using PD-1/PD-L1 antibodies in combination with other drugs on the market or in research: Merck has designed a variety of treatments for small-molecule drugs for Keytruda, with Bristol-Myers Squibb There are hundreds of clinical trials of Opdivo-based combination therapy; Lilly and Cinda, Xinji and Baekje Shenzhou's commercial cooperation involving PD-1 antibodies can also see large pharmaceutical companies deploying tumor immunotherapy, with a view to A determination to get a slice of the future market.
In addition to the combination of PD-1/PD-L1, more immunological checkpoints are becoming a hot target for antibody development. The development of monoclonal antibody drugs such as Tim-3 and LAG-3 is gradually heating up, and more types and more levels of combination drugs are being developed. In the basic research field, the regulation mechanism of immune checkpoints is being continuously explored. The concept of immunization is constantly being deepened. Tumor immunity is moving towards a systematic and refined direction.
With the failure of several clinical trials of single-agent and combination drugs in 2017, the pharmaceutical industry's attitude toward tumor immunity has gradually returned to rationality. The idea of ​​tumor immunity is promising, but people need a lot of time to explore and accumulate in order to truly understand.
China's rapid rise: R&D is gradually at the forefront of the international
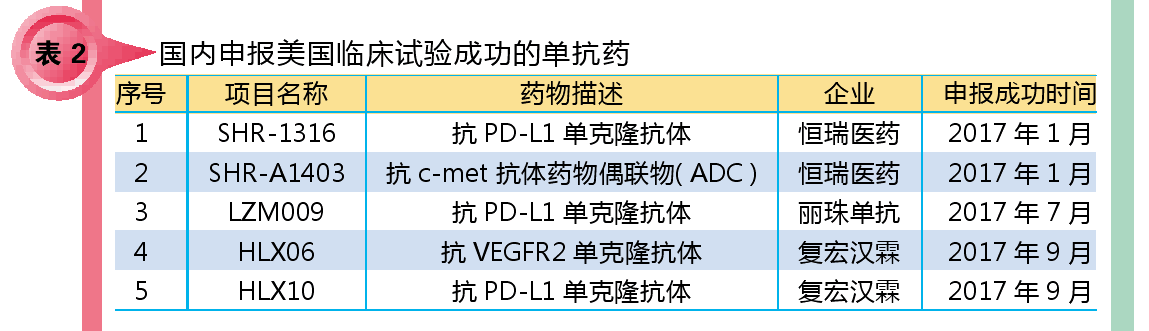
In 2017, a total of five monoclonal antibody drugs were reported to the US for clinical trials. So far, 9 domestic monoclonal antibody drugs have been successfully applied for clinical trials in the United States.
In addition, China Baiji, Cologne medicine, Yu Heng Pharmaceutical and other companies will have their own immune checkpoint inhibitors projects licensed to foreign companies. This information shows that domestic companies are not only getting better results in the field of monoclonal antibody research and development, but also the research and development strength has been internationally recognized.
From the perspective of the monoclonal antibody drug research and development pipelines and declarations of major companies, the R&D layout is concentrated in two aspects:
One is the development of biosimilar drugs for classic drugs. The imitation targets are adalimumab (Xiu Meile), trastuzumab (Herceptin), bevacizumab (Avastin), rituximab. Heavy (Meluohua) and other heavy varieties. The ultimate place for the development of biosimilar drugs is to reduce costs, which in turn reduces drug prices and improves patient accessibility.
The other is the development of immunological checkpoint inhibitors. More concentrated is still the development of antibodies for PD-1/PD-L1. At present, there are more than 10 companies in China with clinical stage projects, and many companies are in the stage of early development and clinical declaration. In addition to these two targets, the research and development of targets such as Tim-3 and LAG-3 have also been rapidly followed up. The re-cooperation between Yuheng Pharmaceutical and Yaoming Biology shows that the domestic research and development progress in the field of tumor immunity has gradually At the forefront of the international arena.
In addition, some companies have deployed technologies such as ADC (such as Hengrui) and bispecific antibodies (such as Jianneng). It can be seen that while doing “me-tooâ€, more and more companies are beginning to try small innovations.
Outlook 2018 : Which new drugs will be approved? What are the new trends?
The author believes that the development of the global monoclonal antibody field in 2018 will follow the trend we have already noticed in 2017 and even earlier. In terms of indications, the field of diseases other than cancer and immune diseases will continue to be exploited, and the application prospects of monoclonal antibody drugs will be further broadened; in terms of technology, mainly bispecific antibodies, antibody drug conjugates (ADCs) and miniaturized antibodies The engineering transformation technology will be continuously developed and applied, and many new molecular designs will be tried in clinical trials.
In the late clinical and drug application stages, I expect the following monoclonal antibody drugs to be approved in 2018:
Taiwan's Zhongyu New Drug TMB-355, an anti-AIDS antibody targeting human immunodeficiency virus (HIV), is currently in the market review stage. Once approved, it will help patients overcome the resistance of traditional antiviral drugs.
Three drugs for the treatment of migraine, namely Erenumab, Eli Lilly, Eli Lilly, and Fremanezumab. It is believed that in 2018, the migraine field will leap from “no effective treatment†to “mass competitionâ€.
Ablynx's Caplacizumab for the treatment of acquired thrombotic thrombocytopenic purpura. If approved, it will be the first approved Nanobody drug.
Abelvi's Rovalpituzumab tesirine, an ADC drug targeting DLL4, is expected to be used to treat small cell lung cancer. Once approved, it will be the fifth approved ADC drug in the world. The consulting firm Evaluate Phama expects the drug to reach $239 million in sales in 2022.
In addition, Sri Lanka's Sirukumab, Concord's fermentation of Kirin's Burusumab, etc. also have great hopes.
As for the research and development layout of domestic monoclonal antibody drugs in 2018, in terms of targets, it will continue to focus on “me-too†and “me-better†in the field of tumor immunity, and other classic targets will continue to receive attention; in terms of technology ADC drugs and bispecific antibodies will not become mainstream quickly, but the number will increase. On the whole, the domestic new drug research and development will be based on “me-too†and “me-betterâ€. Some emerging pharmaceutical companies will try to compare large technological innovations. After the early development of R&D, I believe more companies will Start consciously seeking differentiation.