AstraZeneca's biological respiratory drug Fasenra treats the first phase III clinical failure of COPD
AstraZeneca's biological respiratory drug Fasenra treats the first phase III clinical failure of COPD
May 15, 2018 Source: Sina Pharmaceutical
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];AstraZeneca recently announced top-line data from the phase III clinical study GALATHEA for the treatment of moderate to very severe chronic obstructive pulmonary disease (COPD) with the biological respiratory drug Fasenra (benralizumab). The results showed that the study failed to reach a primary endpoint that resulted in a statistically significant reduction in the severity of COPD. Aggravation of COPD can significantly impair the quality of life of patients, and is associated with disease progression, accelerated decline in lung function, pulmonary hypertension, hospitalization, and mortality; in clinical management, improving lung function, reducing exacerbations, and managing daily symptoms such as difficulty breathing important. In this study, the safety and tolerability of Fasenra was consistent with previous studies. Currently, AstraZeneca is conducting a comprehensive evaluation of the data and the results will be announced at the upcoming medical conference.
This is the first data from Fasenra's treatment of COPD stage III clinical project VOYAGER. The project consists of two Phase III studies, enrolling nearly 4,000 patients, and is the largest COPD biologics development project ever undertaken. In addition to GALATHEA, AstraZeneca is conducting another phase III study, TERRANOVA, which is a randomized, double-blind, 56-week, placebo-controlled, multicenter study that spans a range of baseline eosinophil levels and has a previous condition. Augmented with a history of moderate to very severe COPD patients, Falinera was evaluated as an add-on therapy for double or triple therapy, compared with placebo.
At present, AstraZeneca is awaiting the results of TERRANOVA, and a comprehensive and complete evaluation of these two studies will be conducted to determine the next development strategy for Fasenra treatment of COPD.
COPD is a progressive lung disease that is estimated to affect approximately 384 million people worldwide and is expected to be the third leading cause of death by 2020. At the time of initial diagnosis, about 1/3 were severe or very severe types of COPD. In addition, approximately 30-40% of patients with moderate to severe COPD remain uncontrolled and continue to deteriorate even after receiving triple inhalation therapy (ICS/LAMA/LABA).
Fasenra is a monoclonal antibody drug that targets the alpha subunit (IL-5Rα) of the interleukin 5 receptor on eosinophils and uniquely recruits natural killing cells to induce apoptosis and rapidly deplete Acidic granulocytes, the latter being a white blood cell, is part of the normal composition of the body's immune system. Elevated levels can cause airway inflammation and airway hyperresponsiveness, leading to increased asthma and COPD symptoms, decreased lung function, and acute exacerbation. Raise.
Fasenra is the first respiratory biologic formulation developed by AstraZeneca and the only respiratory biologic therapy that provides direct, rapid, almost complete depletion of eosinophils within 24 hours of dosing. Up to now, the drug has been approved for severe eosinophilic asthma in the United States, the European Union, Japan and several other countries and regions.
IL-15 inhibitor treatment of COPD: GSK stable victory? not necessarily!
Fasenra is also the third IL-5 inhibitor anti-inflammatory drug listed after GlaxoSmithKline (GSK) Nucala and Teva Cinqaero. All three drugs have been approved for the treatment of severe eosinophilic asthma. The latter two target IL-5, in which Nucla is injected subcutaneously once a month, and Cinqaero is infused once a month. These two drugs can lower blood by inhibiting the binding of IL-5 to eosinophil surface receptors. Eosinophil levels in tissues and sputum. While Fasenra is injected subcutaneously every 2 months (8 weeks), IL-5R targeting the surface of eosinophils directly depletes eosinophils.
AstraZeneca believes that Fasenra's combined advantages in efficacy, speed of action, ease of administration, and reduced use of oral steroids will provide a unique treatment option for patients with severe eosinophilic asthma. Previously, AstraZeneca CEO predicted that Fasenra's annual sales peak will reach $2 billion, and investment bank Jefferies analysts gave a figure of $1.5 billion, but this depends on whether Fasenra can successfully develop COPD indications.
In this regard, GSK has taken the lead. In September last year, GSK submitted the NDA for NUCala to treat COPD, but this does not mean that it is stable because its NDA is based on unstable data that is heavily dependent on biomarkers. . In the Phase III study of METREX reported last year, Nucala achieved the goal of reducing the risk of acute exacerbation of moderate to severe COPD, which was 18% lower than placebo and barely reached statistically significant differences, but in the second phase III study METREO Failure to achieve this goal. GSK said that a meta-analysis of the two studies indicated that Nucala may be most effective in patients with COPD with higher eosinophil levels, suggesting that treatment may be required to select the patients most likely to respond to Nucala treatment.
The loss of this study was not the first time Fasenra had treated COPD. As early as in a Phase IIa study in 2014, the drug not only failed to reduce the acute exacerbation rate in patients with severe COPD, but also observed a higher incidence of sudden adverse events. At that time, AstraZeneca executives believed that better trial design and patient selection could bring victory in the field of COPD, which proved to be elusive. There are no new safety or tolerability issues in the GALATHEA study, which is perhaps the only good news in this announcement. The success or failure of the TERRANOVA study is critical to the development of COPD.
AstraZeneca has been in the field of breathing for 40 years and is one of the company's main therapeutic areas. In 2017 alone, more than 17 million people worldwide received treatment for their diverse portfolios. In recent years, AstraZeneca's performance has continued to decline due to patent cliffs and pricing pressures, and Fasenra is one of the top three bio-products that the company hopes to resume its respiratory franchise growth. However, the IL-13 inhibitor tralkinumab has failed in three phase III clinical studies and has been abandoned by AstraZeneca. The other is tezepelumab, an antibody targeting thymic stromal lymphopoietin, developed by AstraZeneca and Amgen. Currently, it is in the phase III clinical development for the treatment of severe asthma. (Sina Pharmaceutical Compilation/newborn)
Article reference source:
1. AstraZeneca provides update on GALATHEA Phase III trial for Fasenra in chronic obstructive pulmonary disease
2, AZ's Fasenra hit by COPD trial failure
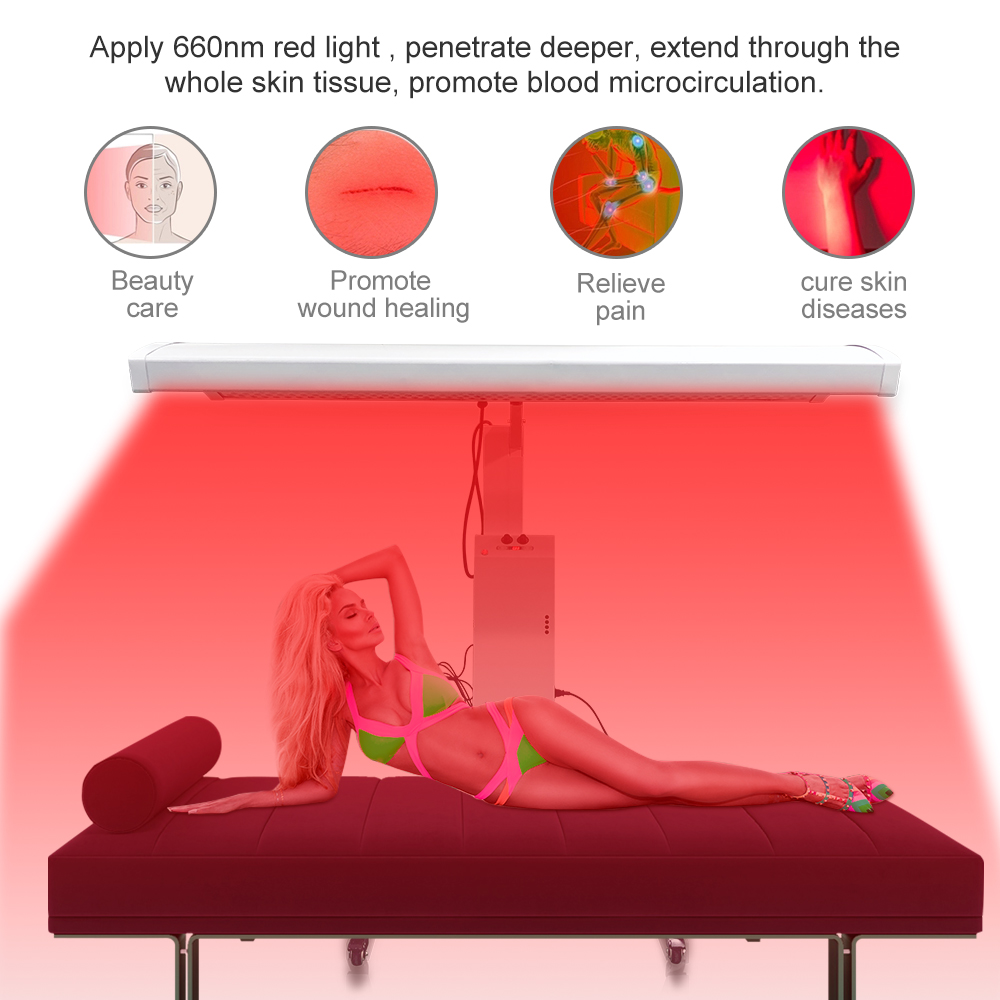
Full Body Red Light Therapy Panel Infrared Light Device Red Light Therapy Panel
Red Light Therapy Panel,red light panel,pdt machine,red light therapy full body,Infrared Light Therapy,Infrared device
Red LED light therapy equipment for plastic surgeons, dermatologists, estheticians, and other licensed skincare and wellness professionals.
Red light therapy has been studied for its potential to improve skin health, reduce inflammation, enhance muscle recovery, and promote overall well-being. It is often used in professional settings, such as spas and wellness centers, but there are also portable at-home versions available for personal use.
Red light therapy panels can be used by positioning them close to the body or by standing in front of them. The treatment duration and frequency vary depending on the desired outcome and the specific device being used. It is generally considered a safe and non-invasive therapy with minimal side effects.
It's important to note that while red light therapy has shown promising results in some studies, more research is needed to fully understand its effectiveness and potential applications. As with any medical or wellness treatment, it is advisable to consult with a healthcare professional before starting red light therapy.
Red Light Therapy Panel,red light panel,pdt machine,red light therapy full body,Infrared Light Therapy,Infrared device
Shenzhen Guangyang Zhongkang Technology Co., Ltd. , https://www.syztreatment.com