Zhejiang Haisheng Medical Device Co., Ltd , https://www.hisernmedical.com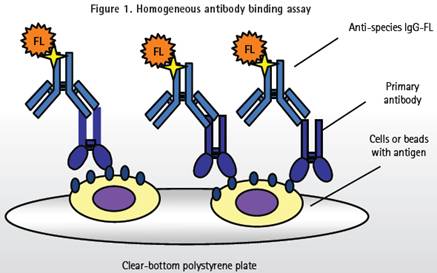
Application of High Connotation Imaging Technology in Monoclonal Antibody Binding Detection-Molecular Devices ImageXpress MicroXLS
Application of high content imaging technology in the detection of monoclonal antibody binding
advantage
1. Detection method without rinsing improves efficiency and reduces waste
2. Wide range of applications, can be used for adherent cells, suspension cells and immunomagnetic beads
3. Fluorescent labels available at any wavelength
4. High sensitivity can detect low abundance antigen
The development of the monoclonal antibody binding assay method has greatly improved the high throughput and high content analysis efficiency of cells and immunomagnetic beads. This method of fluorometric microvolume assay technology (FMAT) can quickly screen for a smaller number of cells without rinsing, and the cell loss caused by multiple plate washing in the traditional sample test can also be completely avoid.
The discovery of antibodies plays an important role in the diagnosis of diseases, the development of vaccines and the treatment of diseases. Researchers used hybridoma cells or clones to screen highly expressed clones, tested antibody binding efficiency on the cell surface, and observed internalization of antibodies or ligands in cells. On top of the existing advantages of traditional FMAT detection methods, the single-antibody binding assay using the ImageXpress® Micro Wide Field High Connotation Analysis System allows scientists to detect surface antibody binding in many different cells in the same plate well. Happening. This system can also increase the scale of detection to 1536-well plates and requires only 2-3 microliters of sample. Even with a lower secondary antibody concentration, the detection range and sensitivity will not decrease or even increase.
Detection principle
The antibody is enriched on magnetic beads and labeled with a fluorescent secondary antibody. Fluorescence imaging technology detects the amount of antibody captured by magnetic beads. The cell surface-bound ligand and the internalization of the ligand and antibody can also be detected by a similar method.
During the monoclonal antibody binding assay (Figure 1), adherent cells, suspension cells or antibody coated magnetic beads samples should be added to 96, 384, 1536 well plates. Then, a primary antibody (target antibody) is added, and then the primary antibody is labeled with a secondary antibody to detect the binding of the primary antibody.
All of the detection reagents were added to the microplate without rinsing. The cell or antibody-coated immunomagnetic beads are immobilized on the bottom surface of the well plate, and a positive signal can be identified and analyzed even if there is high background fluorescence at the bottom of the well plate.
Linearity and detection range
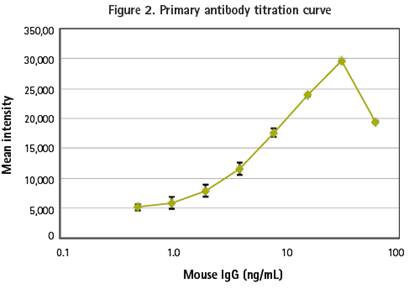
In the first experiment, we titrated different concentrations of primary antibody to bind to 7 micron magnetic beads. The analyte was added, labeled with a secondary antibody and imaged using the ImageXpress Micro High Connotation Analysis System. Traditional FMAT detection methods can only be detected using the red (Cy5) channel, while the ImageXpress Micro system can capture multiple wavelength images, so any secondary antibody tag can be used for marking and detection. In this titration experiment, the secondary antibody was labeled with DyLight 488.
The Image was analyzed using the Count Nuclei module in MetaXpress® high-content image acquisition and analysis software. The lowest detection threshold (LLD) was 0.5 ng/ml (30 pg/well) and the linear response was approximately 1-31 ng/ml. The curve of this primary titration experiment showed "prozone" and the "hook effect" associated with the detection of monoclonal antibody binding. The signal attenuation at the peak of the curve is due to the unbound primary antibody binding to the secondary antibody in the medium, resulting in failure to bind to the cells and magnetic beads (Figure 2).
The primary antibody binding curve of the immunomagnetic beads is shown in the above figure. 12.7 micron coated goat anti-mouse antibody magnetic beads were conjugated to different concentrations of mouse lgG antibody and added to the microplate, and 1-2 incubations in the well plate with the secondary antibody labeled AlexaFluor488 at room temperature Hours, four replicate wells and isotype antibody control wells were set up. The image was taken with a 10X PlanFluor objective. The thumbnail of the well plate (bottom) clearly shows the concentration gradient of antibody binding. Analysis results: LLD was 0.5 ng/mL (30 pg/well), which was linear between 1-31 ng/mL.
Multi-wavelength analysis for more accurate determination of cellular response
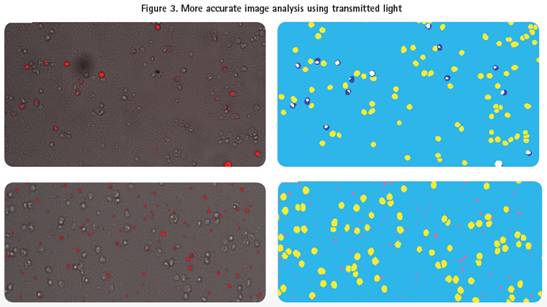
In addition to fluorescent images, the ImageXpress Micro system captures transmitted light (TL) for more accurate cell identification. In this cell-based assay example, the system captures both transmitted and fluorescent images and analyzes the cells using the user-defined module of MetaXpress software.
Fluorescence image observation of the same well showed that the antibody had bound to the cell surface, but if the fluorescence and transmitted light images were superimposed, it can be seen that not every cell is bound to the antibody (Fig. 3, upper left). Subsequent image analysis correctly screened for positive cells bound to the antibody (Figure 3, top right). The Cy5 channel image of the other well shows a high fluorescence signal, indicating an effective binding of the antibody. However, it can be seen from the superposition analysis of transmitted light and fluorescence that the cells in this well are not effectively bound to fluorescence (Fig. 3, bottom left). Image analysis determined that these high fluorescence signals were caused by human factors rather than the cells themselves, so these cells were labeled as not linked to antibodies (Figure 3, bottom right).
Top: Cy5 (red) and transmitted light (translucent) images are superimposed to show positive and negative cells (left) for all antibody binding. Analysis of the figures the bound antibody negative cells displayed a yellow, positive cells (Cy5 channel) is displayed in blue, white overlap (right).
Bottom: Cy5 (red) and transmitted light (translucent) images are superimposed to show the impurity fluorescence (left) that does not overlap with the cells. Negative cells that bind antibody were shown in the analysis as yellow, and cells that were positive for Cy5 channels but not related to cells showed pink (right).
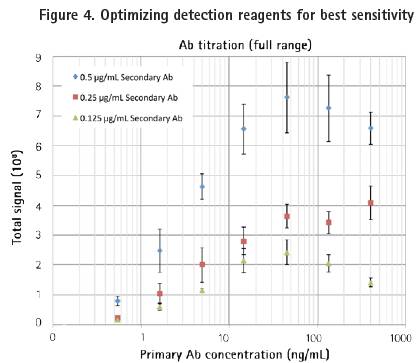
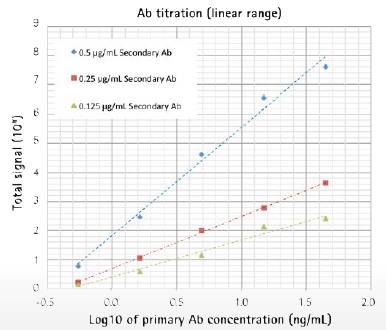
Detection experiments can be easily determined by optimizing the secondary antibody concentration. In this example, we used three different concentrations of secondary antibodies to bind a wide range of primary antibodies. As with the previous magnetic bead test results, the experiment showed a good "hook effect" (Figure 4, top). The minimum detection threshold when the secondary antibody concentration is 0.25 μg/mL is 0.5 ng/ml, which is the same as the standard FMAT method. The three concentrations of the secondary antibody showed good linearity in the titration range (Fig. 4, bottom).
Top: One-effect titration experiment of three different concentrations of secondary antibodies (shown on the X-axis).
Bottom: The results showed that the analyte (primary antibody) showed a linear relationship under a five-fold dilution gradient with an LLD of 0.5 ng/mL.
Image-based monoclonal antibody detection analysis is more flexible
Compared to traditional FMAT detection methods, the use of ImageXpress Micro system and MetaXpress software in combination with high-content imaging analysis methods can provide more information. The system can obtain accurate cell response more quickly on the basis of eliminating the usual human error, thereby obtaining more accurate results. In addition, scientists can detect ligand-cell surface binding and further detect secondary antibody tags at any wavelength, detect different cells on a single plate, and extend detection to 1536-well plates without affecting sensitivity.
The evidence based guidelines shows that Anesthesia breathing circuit components can help to reduce infections,
prevent cross contamination between patients and maintain normothermia.
Why single-use circuits
• Respiratory pathogens can be transmitted through breathing circuits used to provide anesthesia. This can put
reusable circuits at risk for contamination of the next patient.
• The reuse of breathing circuits puts both patients and anesthesiologists at risk for hospital acquired infections.
Disposable circuits can be an important part of prevention protocol.
• The OR has unique infection control issues compared with other clinical care areas-due to the patient`s
vulnerability and prolonged period under anesthesia. Consequently, microorganisms may be transmitted via
contamination of normally sterile sites with a patient`s own bacteria and transmission of bacteria to subsequent
patients in the OR.