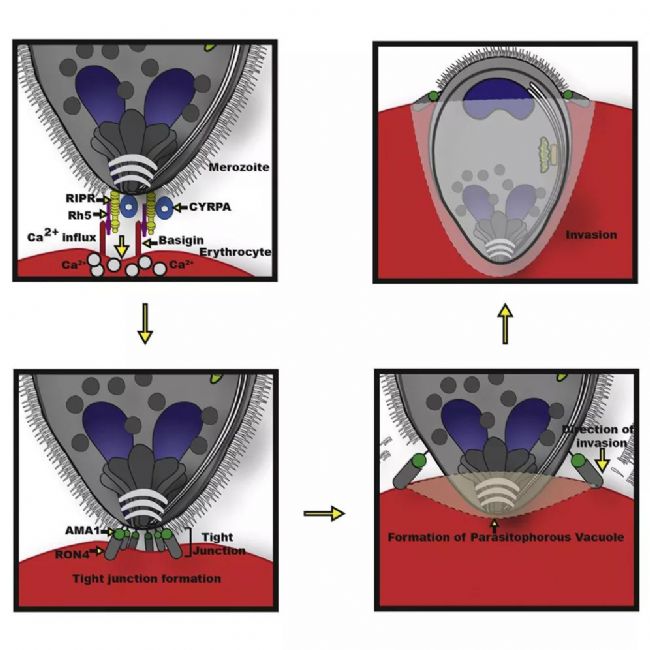
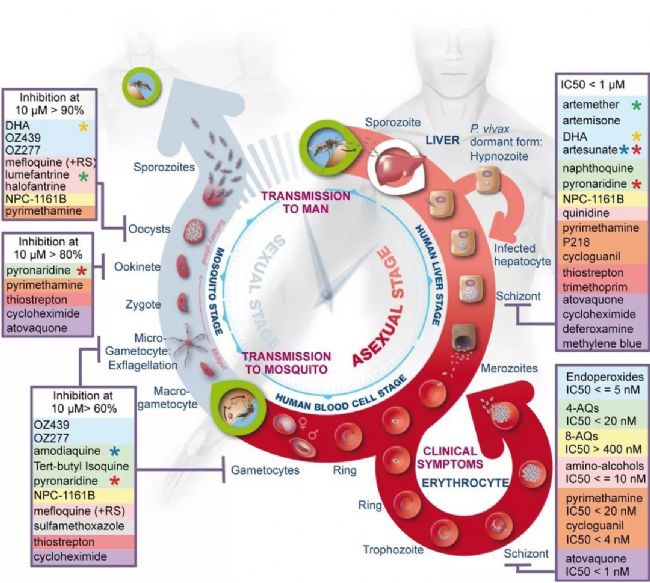
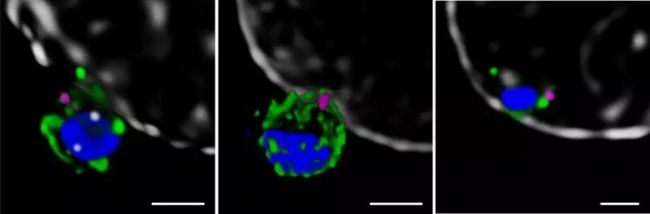
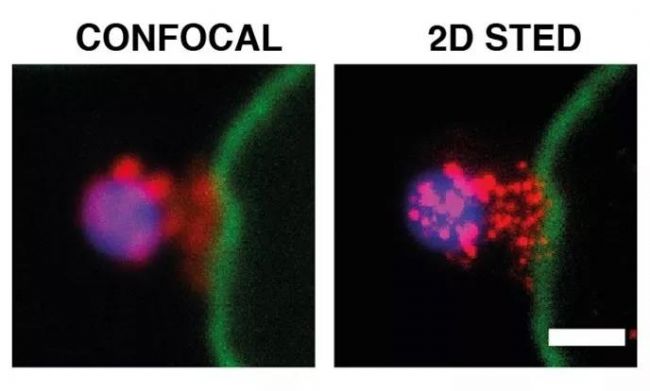
Malaria is a life-threatening disease that spreads through mosquito bites infected with protozoan parasites. The most common and most dangerous type of malaria is malaria caused by Plasmodium falciparum. Malaria raises serious concerns because half of the world’s population is at risk and there is no effective vaccine to date. In 2016, the World Health Organization reported 216 million cases [1], of which 500,000 died, and the economic burden of malaria exceeded billions of dollars. Figure 1. Schematic diagram of Plasmodium invasion of red blood cells At the cellular level, Plasmodium falciparum infection of red blood cells triggers clinical signs of the disease. This phase involves highly dynamic interactions between proteins, which are still poorly understood due to their complexity. Cowman et al., University of Melbourne, Australia, used SP8 STED nanomicroscopes and conditional expression of specific proteins to identify key protein complexes during the invasion phase. The protein forming the complex is capable of linking the host-pathogen, and the presence of the complex is required for host-pathogen tight junctions and erythrocyte Ca2+ release (Figure 1). This breakthrough was published in the journal Cell Host & Microbe [2]. Major participants in malaria infection Figure 2: The three main stages of the life cycle of the Plasmodium: the liver stage, the blood stage and the carrier stage. The green circle shows the key entry point for the parasite from the vector to the host, from the host to the vector. Image source [3]. After entering the human host, with the development of malaria, the malaria parasites exist in different forms during their life cycle (Fig. 2). In the so-called blood stage [4], in the form of merozoites present in red blood cells, through different stages of evolution, about 30 progeny merozoites are produced in infected host cells and are ready to invade other cells. Merozoite invasion is the key to the spread of parasites in the bloodstream and the onset of disease. Despite this, the molecular mechanism of the disease remains unclear. In order to invade red blood cells, merozoites must first contact the host cells, the so-called "pre-invasion" stage [5], which deforms the erythrocyte membrane, and the merozoite ligands bind to specific receptors in the membrane to form a tight junction. Trigger the Ca2+ flow. The flow of Ca2+ seems to be important for cell invasion. The attachment of the parasite to the cells becomes irreversible and enters the red blood cells in less than 2 minutes. Plasmodium falciparum erythrocyte binding protein homolog (PfRh) and erythrocyte binding protein are important signals involved in invasion [6]. A key member of the PfRh family is PfRh5, which binds to the host protein receptor basigin to induce Ca2+ release into erythrocytes and marks the formation of bridging channels between merozoites and blood cell membranes. PfRh5 is critical for merozoite invasion, as shown by studies in which antibodies or soluble basilin inhibit red blood cell infection [7]. PfRh5 works with the PfRh5 interacting protein (PfRipr) and the cysteine-rich protective antigen (CyRPA), but the function of this interaction is unclear. Understanding the role of PfRh5/PfRipr/CyRPA is important because these proteins are important candidates for the development of vaccines that inhibit the development of malaria in the blood [8]. Find the answer to malaria infection with the right tools Video 1 PfRipr-deficient merozoites attempt to infect red blood cells Cowman et al. carried out research work on transgenic Plasmodium falciparum, knocking out the genes of PfRipr and CyRPA, RiprloxCre and CyRPAloxCre [2]. The recombinase (rapamycin-induced DiCre) was added to the parasite containing the RiprloxCre and CyRPAloxCre genes to rapidly and very efficiently regulate gene deletion, thereby controlling the two protein levels accordingly. The authors also performed live cell imaging and confirmed that PfRipr and CyRPA are critical for red blood cell invasion after initial contact with host cells. In the absence of PfRipr or CyRPA, the host cell membrane is still deformed but lacks Ca2+ flow, so the parasite cannot enter the blood cells (Video 1). SP8 STED nano microscope found unknown Next, Cowman et al. studied the localization of PfRh5, PfRipr and CyRPA during the invasion [2]. Confocal imaging showed that the three proteins were preferentially localized at the top of the parasite, which preferentially contacted the host cells. However, accurate assessment of the spatial distribution of biomolecules can only occur at resolution levels below the 3/4 diffraction limit of microscopic imaging, 2D is typically below 30 nm, and 3D STED (excited emission loss) is typically below 100 nm. To this end, red blood cells are incubated with merozoites to induce invasion and target proteins are labeled with antibody probes. Samples were placed on a Leica TCS SP8 STED nanomicroscope for 2D and 3D ultra high resolution imaging (775 nm, video 2). To further determine the initial phase of the invasion, the researchers also immunofluorescently labeled RON4, a protein localized to the apex of the parasite (Figure 3). Figure 3: STED nanodetection of merozoites invading red blood cells. The 3D STED image shows the superposition of RON4 (magenta) with the protein PfRh5 (left, green), PfRipr (medium, green), PfCyRPA (right, green). DAPI nuclear staining is shown in blue and erythrocyte membranes are shown in gray. The scale bar is 1 μm. Image by Professor Walan and Eliza Hall Medical Research Professor Alan Cowman. Video 2 Scheduling invasive red blood cells early STED 3D construction The spatial information obtained with SP8 STED showed that PfRh5, PfRipr and CyRPA were co-localized at the top of the parasite, and the presence of RON4 was also found (Video 2). The single protein and the PfRh5/PfRipr and CyRPA/PfRipr dimer complexes exhibited a splicing distribution on the merozoite membrane, consistent with the release of the apical end. Nearer, higher resolution imaging using 2D STED confirmed the spread of PfRipr and CyRPA on merozoites, but was specifically limited to the apex. Evaluation of the degree of colocalization of PfRh5/PfRipr and CyRPA/PfRipr showed a greater proportion of PfRh5/PfRipr. This finding indicates that CyRPA/PfRipr diffuses onto the merozoite surface, and then the PfRh5/CyRPA/PfRipR complex aggregates directly at the top of the surface to bind to basigin, then triggers the formation of tight junctions and proceeds to the next step in the invading infection. The appearance of individual PfRh5, PfRipr and CyRPA also indicates that each protein can still be used to synthesize complexes. Figure 3: Localization of PfRipr on merozoites that invade red blood cells. Erythrocyte membrane stained with WGA (green). The left is a confocal image, and PfRipr is visualized using 2D STED (MIP). DAPI (blue). The white bars indicate 1 μm. Outlook Invasion of red blood cells is a critical stage in malaria infection in human hosts. Until recently, studies of the interactions and functions of the proteins involved were limited because inhibition of the key ligand PfRh5 completely inhibited the invasion of infection. Using conditioned expression of PfRipr and CyRPA and STED nanomicroscopy, Cowman et al. revealed the important role of PfRipr, CyRPA and PfRh5/PfRipr/CyRPA complexes in merozoite invasion [2]. By the binding of the complex to the host receptor basigin, the protein is involved in the release of Ca2+ into the red blood cells. The SP8 STED nanomicroscope provides the necessary resolution to show the formation of the PfRh5/PfRipr/CyRPA complex at the interface between the parasite and the host cell. Previous studies have shown that CyRPA binds the complex to the parasite membrane [8], but the Cowman group performed additional biochemical analyses and the results showed that this was not the case. Instead, the results indicate that other proteins must anchor the complex to the host cell and direct its formation at the apex. Future work will help identify new proteins involved in merozoite/host cell interactions and understand the functional role of Ca2+ release in the infection pathway. Ultimately, dissecting the interaction between the host and the pathogen will help find a more effective way to fight malaria. references 1. World Health Organization (WHO), World Malaria Report 2017 (WHO, Geneva, Switzerland, 29 November 2017) 2. Volz JC, Yap A, et al. (2016) Essential Role of the PfRh5/PfRipr/CyRPA Complex during Plasmodium falciparum Invasion of Erythrocytes, Cell Host Microbe 20 (1), 60-71, DOI: 10.1016/j.chom .2016.06.004 3. Delves M, Plouffe D, et al. (2012) The Activities of Current Antimalarial Drugs on the Life Cycle Stages of Plasmodium: A Comparative Study with Human and Rodent Parasites. PLoS Med 9(2): e1001169. DOI:10.1371/ Journal.pmed.1001169 4. Cowman, AF, Crabb, BS (2006) Invasion of red blood cells by malaria parasites, Cell 124, 755–766, DOI: 10.1016/j.cell.2006.02.006 5. Weiss GE, Gilson PR, et al. (2015) Revealing the sequence and resulting cellular morphology of receptor-ligand interactions during Plasmodium falciparum invasion of erythrocytes. PLoS Pathog 11 (2), e1004670, DOI: 10.1371/journal.ppat. 1004670 6. Tham WH, Healer J, et al. (2012) Erythrocyte and reticulocyte binding-like proteins of Plasmodium falciparum. Trends Parasitol 28 (1), 23–30, DOI: 10.1016/j.pt.2011.10.002 7. Chen L, Lopaticki S, et al. (2011) An EGF-like protein forms a complex with PfRh5 and is required for invasion of human erythrocytes by Plasmodium falciparum. PLoS Pathog 7 (9), e1002199, DOI: 10.1371/journal .ppat.1002199 8. Douglas AD, Baldeviano GC, et al. (2015) A PfRH5-based vaccine is efficacious against heterologous strain blood-stage Plasmodium falciparum infection in Aotusmonkeys, Cell Host Microbe 17 (1), 130–139, DOI: 10.1016/j.chom.2014.11.017 9. Reddy KS, Amlabu E, et al. (2015) Multiprotein complex between the GPI-anchored CyRPA with PfRH5 and PfRipr is crucial for Plasmodium falciparum erythrocyte invasion, Proc. Natl Acad Sci USA 112 (4), 1179–1184, DOI: 10.1073/pnas.1415466112 About Leica Microsystems Leica Microsystems Leica Microsystems is a global leader in microscopy and analytical science instruments and is headquartered in Wetzlar, Germany. It mainly provides professional scientific instruments such as research-grade microscopes in the field of microstructure and nanostructure analysis. Since the establishment of the company in the 19th century, Leica has been widely recognized by the industry for its quest for optical imaging and its innovative spirit of continuous improvement. Leica is a global leader in multiple microscopy in composite microscopes, stereo microscopes, digital microscopy systems, laser confocal scanning microscopy systems, electron microscopy sample preparation and medical surgical microscopy. Leica Microsystems has seven product development and production sites around the world and has service support centers in more than 20 countries. Leica has regional branches or sales branches in more than 100 countries around the world, and has established a comprehensive dealer service network system throughout the world. Calcium Hydroxide,Slaked Lime,Hydrated Lime,Food Grade Calcium Hydroxide Wuxi Yangshan Biochemical Co.,Ltd. , https://www.salesacetates.com