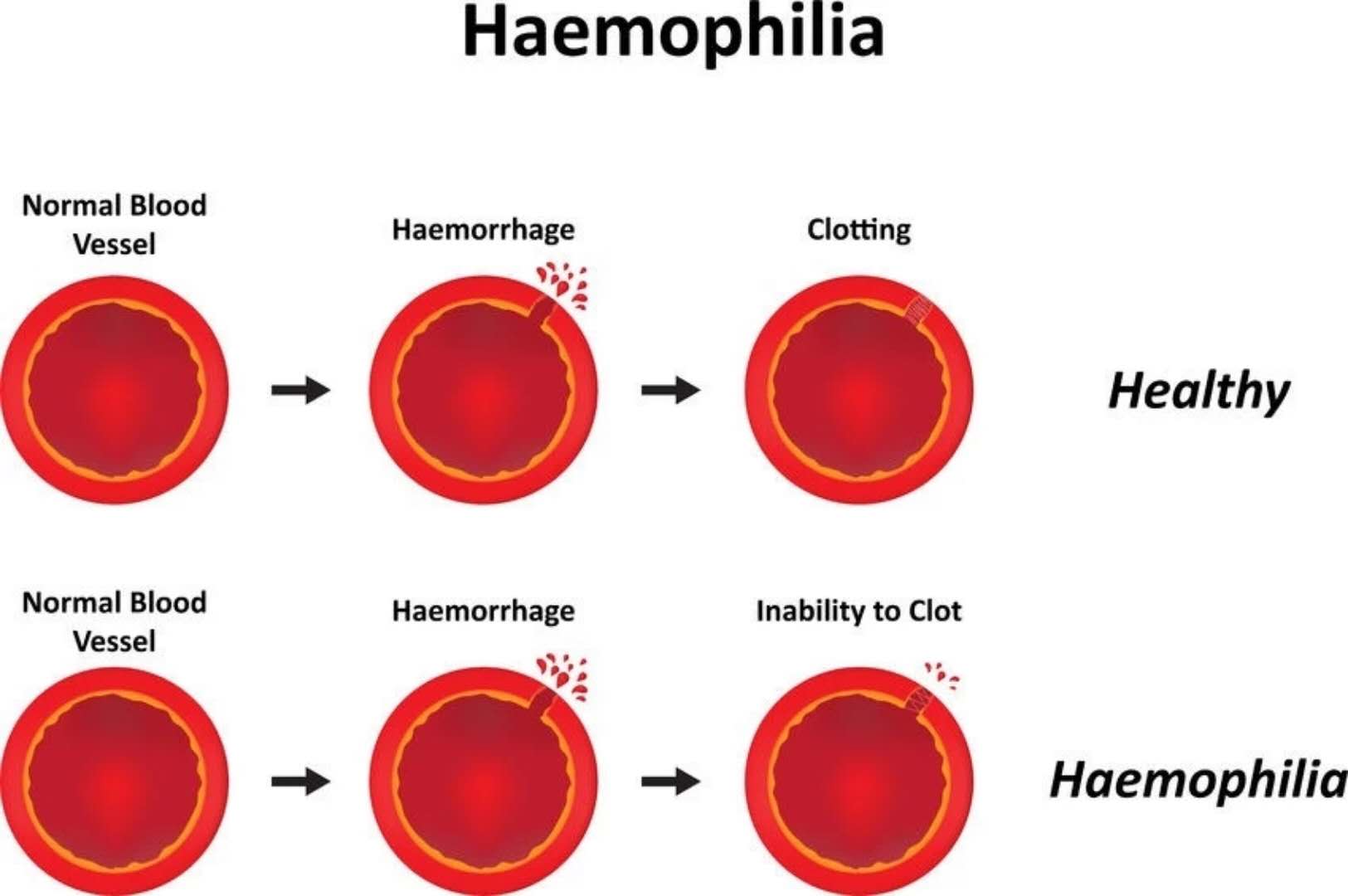
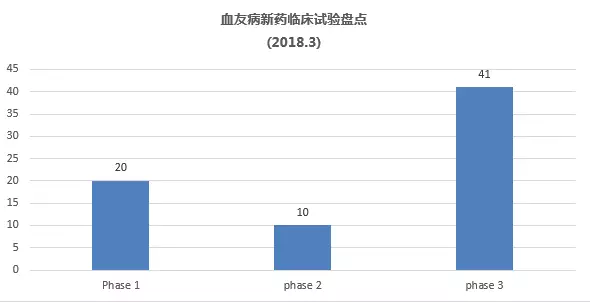
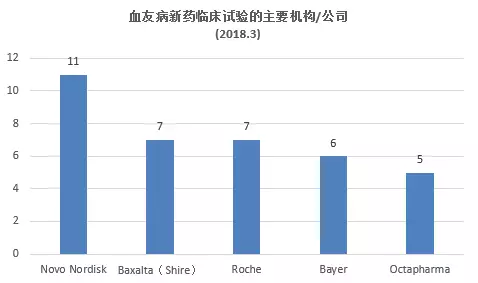
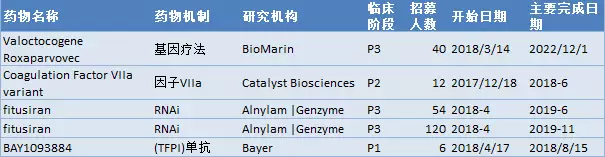
What are the latest hemophilia R&D pipelines in March 2018? April 23, 2018 Source: WuXi PharmaTech Hemophilia is an inherited bleeding disorder. The patient does not produce enough thrombin to ensure effective blood clotting, resulting in repeated bleeding of muscles and major organs. â–² abnormal blood coagulation in patients with hemophilia (Source: 123RF) There are two main types of hemophilia: hemophilia A, caused by insufficient factor VIII; hemophilia B, caused by insufficient factor IX. Other types include hemophilia C, which is caused by a deficiency in factor XI. The incidence of hemophilia A is about one in every 5,000-10,000 people, and about one in every 40,000 people with hemophilia B. Since A/B hemophilia is X-chromosome recessive, the patient is mainly male. Hemophilia type C has the same incidence in both sexes, most of which occurs in German Jews. Approximately 43% of patients with hemophilia A are of a severe type, who often spontaneously bleed and flow into muscles or joints. Currently, the standard treatment for these patients is a three-week infusion of factor VIII for prophylactic treatment. Prophylactic or "on-demand" clotting factor input can temporarily restore blood clotting capacity. However, patients with hemophilia have a risk of developing neutralizing antibodies or replacement factor "inhibitors" that affect approximately one-third of patients with hemophilia A and some patients with hemophilia B, for the production of replacement factors. In patients with inhibitors, the chances of hospitalization due to bleeding time doubled. These patient groups are in desperate need of a new treatment to change the status quo. Gene therapy is currently being studied and is expected to reduce the severity of severe hemophilia to mild or moderate levels. This article takes stock of the latest clinical development pipeline for hemophilia in March 2018. We collected clinical trial data for hemophilia drugs in Phase 1, Phase 2, and Phase 3, registered on ClinicalTrials.gov. Included in the statistics are trials that are being recruited, recruited, and issued recruitment notices, and do not include trials that have been completed, terminated, unknown, withdrawn, and suspended. New drug pipeline inventory in the clinical stage of hemophilia As of February 28, 2018, there were 71 clinical trials of new drugs in the field of hemophilia, and late clinical studies dominated. These include: 20 trials in Phase 1, including 10 small molecule drugs and 10 biopharmaceuticals; 10 trials in Phase 2, including 5 small molecule drugs and 5 biopharmaceuticals; Forty-one trials, including 22 small molecule drugs and 19 biological drugs (note: some drugs may be tested simultaneously). Clinical trials of new hemophilia drugs are concentrated in the United States and Asia. According to the location of the research institution of the clinical trial, the top 5 countries or regions are: 50 trials in the United States, 31 in the United Kingdom, 28 in Spain, 27 in Italy, and 25 in France (Note: There may be multi-center clinical trials). The top 5 companies/research institutions with the largest number of clinical trials of hemophilia new drugs are: Novo Nordisk 11 trials, Baxalta (Shire) 7 trials, Roche 7, Bayer 6 and Octapharma 5 trials. New clinical trial of new hemophilia drugs in 2018 In 2018, five new clinical trials of hemophilia were added. One phase 1 trial enrolled 6 subjects, one phase 2 trial enrolled 12 subjects, and three phase 3 trials enrolled an average of 71 subjects. â–²A new clinical study on new hemophilia drugs in 2018 It is worth noting that two hemophilia gene therapies entered Phase III clinical trials, namely BioMarin's valoctocogene roxaparvovec and Alnylam's RNAi therapy Fitusiran. Bayer's BAY1093884 is a fully humanized monoclonal antibody against tissue factor pathway inhibitor (TFPI) with a novel mechanism of action and a phase 1 clinical trial. One of the factors VIIa has the advantage of prophylactic subcutaneous injection in the research of new drugs, and is currently in phase 2 clinical. The following is an introduction to the new hemophilia clinical trial and new drug research this year. New drug name: Valoctocogene Roxaparvovec Indications: hemophilia A Drug Mechanism: Gene Therapy Research institute: BioMarin Pharmaceutical Clinical stage: Phase 3 BioMarin's valoctocogene roxaparvovec is a gene therapy product using the AAV Factor VIII vector. In animal models, it restores the concentration of Factor VIII to levels comparable to normal humans. The drug was approved by the FDA for breakthrough therapy and the PRIME certification by the European Medicines Agency (EMA). Roxaparvovec is a gene therapy for AAV vectors that contains the correct genes to make Factor VIII, which restores the patient's own Factor VIII function to normal. This is a phase 3 study evaluating the safety and efficacy of the gene therapy roxaparvovec for the treatment of patients with severe hemophilia A at 4E13 vg/kg. The purpose of this study was to understand whether hepatocytes were able to normalize factor VIII after administration; how much factor VIII concentrate was injected after administration and the effects of bleeding events; and whether and how the body responded to the study drug - For example, whether liver cells are inflamed, or whether the body produces antibodies (the immune system protects itself from substances such as bacteria and viruses) or the new Factor VIII gene. The trial program enrolled 40 subjects. The expected start date for the study was March 14, 2018, and the expected major completion date was December 1, 2022. New drug name: Fitusiran Indications: A/B hemophilia Drug Mechanism: RNAi Therapy Research institute: Alnylam | Genzyme Clinical stage: Phase 3 Fitusiran is one of Alnylam's main products, an RNAi therapy for anti-thrombin, which is administered subcutaneously once a month for the treatment of hemophilia A and B. This method aims to reduce the level of antithrombin so that there is enough thrombin in the body to stop bleeding and prevent bleeding. In a clinical study in 2017, a clinical trial of utilis iran for hemophilia was once stopped by the FDA because a patient with hemophilia A died after receiving treatment with fitusiran. In December 2017, the US FDA approved the restart of fitusiran's clinical research on hemophilia, including the Phase 2 Open Label Extension Study and the Phase 3 Research Program ATLAS. This year, Fitusiran added two new phase 3 clinical studies. The ATLAS-INH study recruits patients with hemophilia A/B who are resistant to factor VIII or IX, and ATLAS-A/B recruits patients with hemophilia A/B who are not resistant to factor VIII or IX. Both clinical studies will assess the efficacy and safety of the treatment of fitusiran, as compared to conventional medications. The ATLAS-INH trial enrolled 54 subjects. The expected study start date is April 2018 and the expected major completion date is June 2019. The ATLAS-A/B pilot program enrolled 120 subjects. The expected study start date is April 2018 and the expected major completion date is November 2019. New drug name: Marzeptacog Alfa Indications: A/B hemophilia Drug Mechanism: Factor VIIa Research institute: Catalyst Biosciences Clinical stage: Phase 2 Marzeptacog alfa (activated) is an effective new generation factor VIIa for effective, long-term prophylactic inhibitor prevention in patients with hemophilia. It is designed to combine higher blood clotting activity and longer activity in the bleeding site to improve efficacy. The potential of Marzeptacog alfa is expected to be used for subcutaneous prophylactic treatment. The US FDA has granted marzeptacog alfa orphan drug status for the treatment of hemophilia A and B. The newly registered subcutaneous efficacy test in 2018 is an open-label, dose-increasing study evaluating the efficacy and safety of daily subcutaneous injection of Marzeptacog Alfa to prevent bleeding in adults with type A or B hemophilia. The primary endpoint is to reduce the annual bleeding rate, which will be used as a control for each person's historical annual bleeding rate. This trial will enroll 12 adult males with severe hemophilia A or B hemophilia at approximately 10 clinical trial sites around the world. The patient had a history of frequent bleeding events during the first 6 months prior to enrollment, administered by intravenous and subcutaneous injections based on individual bleeding and treatment records. The expected study start date is December 18, 2017 and the expected major completion date is June 2018. New drug name: BAY1093884 Indications: severe hemophilia Drug Mechanism: Tissue Factor Pathway Inhibitor (TFPI) Monoclonal Antibody Research institution: Bayer Clinical stage: Phase 1 BAY1093884 is a fully humanized monoclonal antibody directed against tissue factor pathway inhibitor (TFPI) that acts as a bypass agent for hemophilia patients with or without inhibitors. It restores the deficiency of thrombin, forms a stable blood clot, and effectively prevents bleeding in the body. The primary objective of this study was to evaluate the pharmacokinetics of BAY1093884 in the treatment of patients with severe hemophilia, with the secondary goal being pharmacodynamics. The trial plan recruited six subjects. The expected study start date is April 17, 2018. The expected major completion date is August 15, 2018. Note: The title map source 123RF Reference materials: [1] ClinicalTrials.gov [2] Company's official website [3] Green light is released! Hemorrhagic disease RNAi therapy restart clinical trial [4] Hemophilia gene therapy is recognized by the US FDA for breakthrough therapy
PVC
Coated Wiggle Wire will help to ensure that your greenhouse can weather the
elements and last for years. Unlike other attachment methods, Wiggle Wire will
hold up to high winds and harsh environments. Protect your investments by using
the right products. The PVC Coated Wiggle Wire prevents tears for a fast
frustration free installation. This Spring Wire is the superior choice of
Wiggle Wire compared to the non-coated version. The PVC coating prevents tears
and is easier to handle. It is reusable and can easily be installed and removed
by "wiggling" it in and out of the Lock Channel. For use with poly
greenhouse film, curtains, shade cloth and other greenhouse coverings.
Plastic Coated Steel Wire,Plastic Coated Wire,Pvc Coated Wire Mesh,Plastic Coated Wire Mesh JIANGSU SKYPLAN GREENHOUSE TECHNOLOGY CO.,LTD , https://www.greenhousehydroponic.com